Herb Identification and Authentication
Have you ever wondered how we determine if the herbs that we are supplying you for your patients are the correct species? That they are not inferior or toxic herbs that are being substituted for the textbook pinyin-named herbs? How do we ensure the herbs that you are buying are medicinal rather than agricultural or food grade? This month, I want to explain how Mayway establishes its identity and quality standards for its Plum Flower® Premium QualityTM herbs.
The herbal marketplace within China is vast and, reportedly, over 7,000 substances: botanical, zoological, and mineral are sold as “herbs” or “crude drugs”. There are some 600 centers within China where herbs make up a large portion of the local economy and 17 of China’s 22 provinces have major herbal markets including notable ones: Qi Zhou in Anguo, Hebei; He Hua Chi in Chengdu, Sichuan; Qing Ping in Guangzhou, Guangdong; Bo Zhou in Anhui; and Yu Zhou in Henan. The volume of the herb market in China was estimated at US $14 billion in 2005 and is expected to grow to US $24 billion by 2014.
The various regions of China all lay claim to certain dao di 道地 or geo-authentic herbs that grow best in that region. This is analogous to terroir which refers to the special characteristics that the geography, geology and climate of a certain place provide for particular varieties of wine, coffee, and tea. However, nearly all regions in China grow many other important herbs for sale in the global market that are zheng yao 正药, that is, the standard, “correct”, medicinal species of the official Chinese herbs according to the People’s Republic of China (PRC). They also grow local variants that some call di fang yong yao 地方用藥 or “medicine used regionally”, which may be a local alternative or substitute species. In other cases, these local variants are simply inferior species or non-medicinal grade herbs. Mayway has noticed, both in markets in China and the US, herbs such as “Shan yao” and “Gou qi zi” being sold for botanical medicine that are actually only food grade. Unfortunately, some of the local variants also have radically different actions and/or may contain chemicals such as aristolochic acid or pyrrolizidine alkaloids that are toxic and that are not present in the standard herbs.
Thus, the question of authenticity and the certification of identification has become an issue of paramount importance. Mayway obtains its herbs from all over China and then they are brought to our joint-venture facility, Mayway Hebei in Anguo, Hebei, PRC. There, the on-site Quality Control Department performs the appropriate organoleptic, macroscopic, microscopic, and/or analytic testing to ascertain the identity of every herb that we sell.

The Pharmacopoeia of the People’s Republic of China has been published since 1953 and contains the official general scientific and technical provisions for drug quality control and administration. Volume I of the Pharmacopoeia consists of monographs in three sections: (1) “Chinese Materia Medica and Prepared Slices of Chinese Crude Drugs”[sic—the PPRC refers to herbs as “crude drugs” or simply, “drugs”]; (2) “Oils, Fats and Extractives”; and (3) “Traditional Chinese Patent Medicines and Simple Preparations”. At the end of the book are 17 Appendices which provide official definitions of terms and detailed analytical methods to be used in the performance of procedures and assays defined in the monographs in the main text. The 2010 PPRC contains 4567 monographs including 2165 monographs on individual herbs and herbal formulas in Volume I. Within the monographs are described over 1500 thin layer chromatography tests (TLC) and over 800 high performance liquid chromatography tests (HPLC). In conjunction with our manufacturing partners in China, we use Part 3 to determine the production specifications of our herb formula products. For authentication and to determine the identity of our herbs, we use Part 1.
The monograph of each herb/drug in the PPRC includes the following information: (1) the Chinese character name, pinyin name, Latin name and English name; (2) source information as to from where the herb is derived and other information such as harvest season or handling methods at harvest such as drying methods or initial cleaning; (3) formulary—a specification by which medicines are approved to be prescribed. The development of the formulary is based on evaluations of efficacy, safety, and cost-effectiveness of drugs and contains additional clinical information, such as actions, indications, side effects, precautions, contraindications, dosages, and storage; (4) processing—not only the common standards such as “soak and cut into thin slices before final drying” or “dry in the sun” , but also including methods such as honey frying, charring, calcining, et al.; (5) description—including organoleptic and macroscopic descriptions, as appropriate, and references to the appearance, texture, cross section, odor, taste, solubility, and other physical constants of the correct species of the herb; (6) identification—including detailed microscopic and analytical testing usually using HP-TLC in comparison to a Reference Standard; (7) inspection criteria of analytical testing—including total ash, water content and impurities; (8) extractive levels of certain known ingredients; and (9) assay method for known ingredients—usually using HPLC, GC, or GC-MS, as applicable, again, in comparison to a Reference Standard.
Chemical reference substances, reference drugs, reference extractives, and reference standards refer to the standard materials used in identification testing, and other tests and assays. Those are established, standardized, and distributed to designated, approved testing laboratories by the drug regulatory authority of the PRC State Council, State Food and Drug Administration (SFDA). That is to say, only the reference standards that are obtained from the SFDA are considered authoritative. Moreover, the drugs and preparations are required to be tested with official methods as stated in the Pharmacopoeia. However, the PRC State Food and Drug Administration allows the application of alternative methods, if they have been proven to perform satisfactorily in comparison with official methods. In case of doubt or dispute, the methods of Pharmacopoeia are otherwise authoritative. A good example of this is in testing for sulfur dioxide, our lab uses a test method that is an approved modification of the published method in the PPRC.
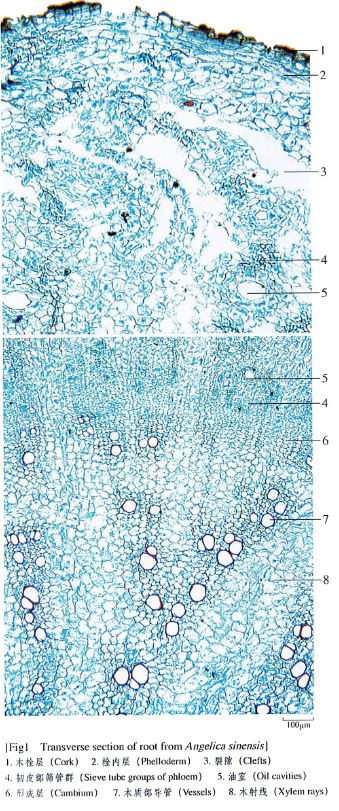
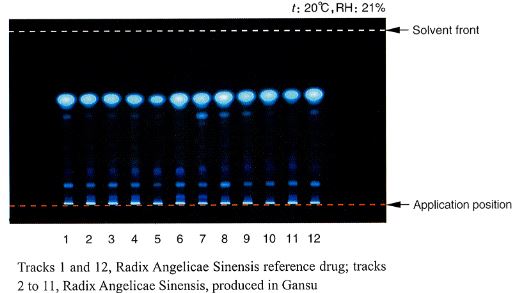
To illustrate how the monographs in the Pharmacopoeia define an herb completely, the documentation regarding Radix Angelicae Sinensis (dang gui 当归) is reprinted here, in its entirety along with a figure that illustrates the microscopic examination and a figure that describes the thin layer chromatography testing of dang gui.
Radix Angelicae Sinensis (当归, Danggui)
Chinese Angelica
Chinese Angelica is the dried root of Angelica sinensis (Oliv.) Diels (Family Umbelliferae). The drug is collected in late autumn, removed from rootlet and soil, slightly dried and tied up in small bundles, placed on a shelf and smoke-dried.
Description
Somewhat cylindrical, 3-5 or more branched at the lower part, 15-25 cm long. Externally yellowish-brown to brown, longitudinally wrinkled and transversely lenticel-like protruded. Root stocks (Guitou ) 1. 5-4 cm in diameter, annulated, apex obtuse and rounded, showing purple or yellowish-green remains of stems and leaf sheaths; main roots (Guishen) lumpy on the surface; branching” roots (Guiwei) 0.3-1 cm in diameter, the upper portion thick and the lower portion thin, mostly twisted and exhibiting a few rootlet scars. Texture flexible, fracture yellowish-white or yellowish-brown, bark thick, showing some clefts and numerous brown dotted secretory cavities, wood paler in colour, cambium ring yellowish-brown. Odor, strongly aromatic; taste, sweet, pungent and slightly bitter.
It is not used medicinally if the roots become woody, withered and not oily, or greenish-brown on the fracture.
Identification
Microscopic (See fig. 1)
(1) Transverse section: Cork cells in several layers. Phelloderm narrow, scattered with a few oil cavities. Phloem broad, more cleft, oil cavities and oil tubes subrounded, 25-160 μm in diameter, relatively large on the outer side, gradually becoming small inwards, surrounded by 6-9 secretory cells. Cambium in a ring. Xylem rays 3-5 rows of cells wide; vessels singly scattered, or 2-3-grouped, arranged radially; parenchymatous cells containing starch granules.Powder: Yellowish-brown. Parenchymatous cells in phloem fusiform, walls slightly thickened, with very fine oblique crisscross striations, sometimes thin transverse septa visible. Scalariform and reticulate vessels frequent, up to 80 μm in diameter. Sometimes fragments of oil cavities visible.
HP-TLC (See fig. 2)
(2) To 0.5 g of the powder add 20 ml of ether, ultrasonicate for 10 minutes and filter. Evaporate the filtrate to dryness and dissolve the residue in 1 ml of ethanol as the test solution. Prepare a solution of 0. 5 g of Radix Angelicae Sinensis reference drug in the same manner as the reference drug solution. Carry out the method for thin layer chromatography (Appendix VI B), using silica gel G as the coating substance and a mixture of n-hexane and ethyl acetate (4:1) as the mobile phase. Apply separately to the plate 10 μl of the above two solutions. After developing and removal of the plate, dry in air. Examine under ultraviolet light at 365 nm. The fluorescent spots in the chromatogram obtained with the test solution correspond in position and color to the spots obtained with the reference drug solution.
(3) To 3 g of the powder add 50 ml of 1% solution of sodium bicarbonate, ultrasonicate for 10 minutes, and centrifuge. Adjust the supernatant to pH 2-3 with dilute hydrochloric acid, extract by shaking with two 20-ml quantities of ether, combine the ether extracts and evaporate to dryness. Dissolve the residue in 1 ml of methanol as the test solution. Dissolve a quantity of ferulic acid CRS in methanol to produce a solution containing 1 mg per ml as the reference solution. Carry out the method for thin layer chromatography (Appendix VI B), using silica gel G as the coating substance and a mixture of benzene, ethyl acetate and formic acid (4:1:0.1) as the mobile phase. Apply separately to the plate 10 μl of each of the above two solutions. After developing and removal of the plate, dry in air. Examine under ultraviolet light at 365 nm. The fluorescent spot in the chromatogram obtained with the test solution corresponds in position and color to the spot obtained with the reference solution.
Water
Carry out the method for determination of water (Appendix IX H, method 2), not more than 12%
Ash
Not more than 7% (Appendix IX K).
Acid-insoluble ash
Not more than 2. 0 per cent (Appendix IX K).
Extractives
Carry out the method for determination of ethanol-soluble extractives (Appendix X A, the hot extraction method), using 70% ethanol as the solvent, not less than 45.0 per cent.
Assay
Carry out the method for high performance liquid chromatography (Appendix VI D).
Chromatographic system and system suitability: Use octadecylsilane bonded silica gel as the stationary phase and a mixture of acetonitrile and 0.085% solution of phosphoric acid (17: 83) as the mobile phase. As detector a spectrophotometer set at 316 nm, maintain the column temperature at 35°C. The number of theoretical plates of the column is not less than 5000, calculated with the reference to the peak of ferulic acid.
Reference solution: Weigh accurately 10 mg of ferulic acid CRS, dissolve in 70% methanol in a 50 ml brown volumetric flask and dilute to volume. Measure accurately 3 ml in a 50 ml brown volumetric flask, dilute with 70% methanol to volume to produce a solution containing 12 μg per ml, and mix well.
Test solution: Weigh accurately 0. 2 g of the powder (through No. 3 sieve ) to a stopper conical flask, accurately add 20 ml of 70% methanol, stopper tightly and weigh. Heat under reflux for 30 minutes, cool and weigh again, replenish the loss of solvent with 70% methanol, mix well and stand. Filter the supernatant through the millipore (0. 45 μm), use the successive filtrate as the test solution.
Procedure: Accurately inject 10 μl of each of the reference solution and the test solution, respectively, into the column, and calculate the content. It contains not less than 0.050 per cent of ferulic acid (C10H10O4), calculated with reference to the dried drug.
Processing
Radix Angelicae Sinensis: Eliminate foreign matter, wash clean, soften thoroughly, cut into thin slices, and dry in the sun or at a lower temperature.
Radix Angelicae Sinensis (stir-baked with wine): Stir-bake the slices of Radix Angelicae Sinensis as described under the method for stir-baking with wine (Appendix II D) to dryness.
Subround or irregular slices, with brown rings in the cut surface. Texture flexible, deep yellow in color, with burnt stains.
Taste: sweet, slightly bitter; odor: strongly aromatic and wine-like.
Action
To enrich blood, activate blood circulation, regulate menstruation, relieve pain, and relax bowels.
Radix Angelicae Sinensis (stir-baked with wine): To activate blood circulation and stimulate menstruation.Indications: Anemia with dizziness and palpitation; mens trual disorders, amenorrhea, dysmenorrhea; constipation; rheumatic arthralgia; traumatic injuries; carbuncles, boils and sores.
Radix Angelicae Sinensis (stir-baked with wine): Amenorrhea, dysmenorrhea, rheumatic arthralgia, traumatic injuries.
Usage and dosage 6-12 g.
Storage Preserve in a cool and dry place, protected from moisture and moth.
---
By using such a reference standard as the Pharmacopoeia of the People’s Republic of China for each of our herbal products, I am sure you can see that Mayway aspires to the highest standard of quality assurance available in the People’s Republic of China. Not only do we insist that every herb that we sell is the approved herb by the PRC (there are many herbs not listed in the PPRC, and then the Zhong Hua Ben Cao is accepted as an official reference) but we also test the identity of each herb that arrives at Mayway Hebei using the appropriate analytical methods that establish, without equivocation, the authenticity of the herb. Further, Mayway has created a Product Specification Sheet that describes each of our products and that lists all tests, methods, and testing limits performed on a particular product.
Finally, every batch of each of our products that is received at Mayway USA is accompanied by a Certificate of Analysis (available upon request) upon which is published the results of all tests performed on that product, including identity testing, and which confirms that our specifications have been met.
References
- An Illustrated Handbook on Microscopic Identification of Chinese Crude Drugs in Pharmacopoeia of the People’s Republic of China, State Pharmacopoeia Commission of the PRC, People’s Medical Publishing House, Beijing, 2009.
- European Pharmacopoeia, European Directorate for the Quality of Medicines (EDQM), Council of Europe, Strasbourg, FR 2011. http://www.edqm.eu/en/European-Pharmacopoeia-1401.html
- Pharmacopoeia of the People’s Republic of China, State Pharmacopoeia Commission of the PRC, People’s Medical Publishing House, Beijing, 2005, 2010.
- TLC Atlas of Chinese Crude Drugs in Pharmacopoeia of the People’s Republic of China, Peishan Xie, Ed., State Pharmacopoeia Commission of the PRC, People’s Medical Publishing House, Beijing, 2009.
- The United States Pharmacopeial Convention, http://www.usp.org/
- Traditional Chinese Medicine (TCM) In China and Worldwide 2010-2011-2025: Markets, products, companies, Developments, Technologies and Sciences October 2011, Helmut Kaiser Consultancy, http://www.hkc22.com/chinesemedicine.html
- Zhong Hua Ben Cao, Editor: Ying Xiao-hung, et al., Shanghai Scientific & Technical Publishers, Shanghai, 1999.
Copyright © Mayway Corp. 2011. All rights reserved.


